Steam tables

good evening all,
Maybe someone could help with the following question; the dynamic viscosity of water changes with temperature (known) however does it change with
pressure? i need to know the dynamic viscosity of water @110 celsius 7Bar pressure
cheers
I can't see why it should vary with pressure - 7 bar is not that exotic. I assume you mean kinematic viscosity (visc/density). OK density will
increase ever so slightly with presure.
And if you need it for doing some kind of hydrodynamic simulation other errrors & assumptions will dwarf any physical constant errors!
quote:
Originally posted by luke_stephenson
good evening all,
Maybe someone could help with the following question; the dynamic viscosity of water changes with temperature (known) however does it change with pressure? i need to know the dynamic viscosity of water @110 celsius 7Bar pressure
cheers
I was reading an article about this the other day in New Scientist. I think it does change but I can't say for sure.
does this help
http://www.engineeringtoolbox.com/water-dynamic-kinematic-viscosit
y-d_596.html
quote:
Originally posted by Nash
quote:
Originally posted by luke_stephenson
good evening all,
Maybe someone could help with the following question; the dynamic viscosity of water changes with temperature (known) however does it change with pressure? i need to know the dynamic viscosity of water @110 celsius 7Bar pressure
cheers
It's steam at 110 deg C and 7 bar?!?!?!?
...........Neil

quote:
Originally posted by Nash
It's steam at 110 deg C and 7 bar?!?!?!?
...........Neil
quote:
Originally posted by flak monkey
quote:
Originally posted by Nash
It's steam at 110 deg C and 7 bar?!?!?!?
...........Neil
Nope still a liquid I think at that pressure and temperature.
with ref to engineers toolbox, thankyou, id already checked there tho. v.good site!
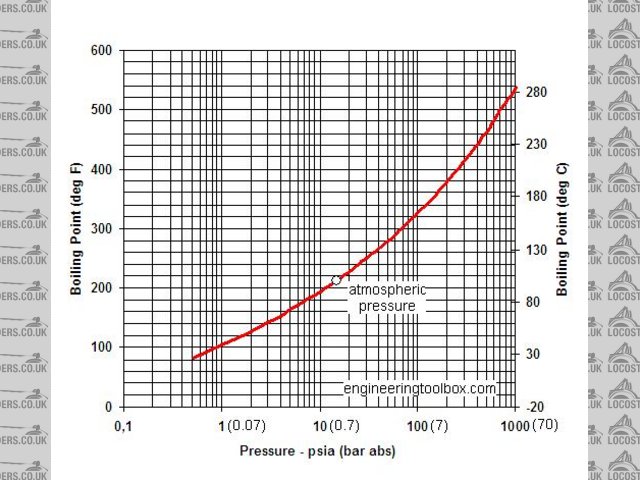
afraid its definately still in the liquid state, saturation temp @ 7 bar is 165celsius.
ok so this is my theory, as shown in the engineers toolbox and other sites the dynamic viscosity which is different to kinematic viscosity varies with
temperature @ atmospheric pressure. however the chart only goes to 100c obviously as the water will boil. and it is shown that as the water nears
boiling point the dynamic viscosity lowers.
all well and good, but the 7bar pressure would effectively have the water in a totally different state as it would not be near boiling point. its got
me well stumped.
unfortunately i need it to get a reliable reynolds number to allow accurate heat transfer calcs and more importantly accurate scaling of the proposed
heat exchanger.
im well and truely stuck!
thanks for all the input so far
quote:
Originally posted by will121
quote:
Originally posted by flak monkey
quote:
Originally posted by Nash
It's steam at 110 deg C and 7 bar?!?!?!?
...........Neil
Nope still a liquid I think at that pressure and temperature.
definately still be a liquid, cant get my steam tables out of the loft be covered in 20years of dust, but we run high temp Hot water heating systems upto 150degC @10bar,


This is where I normally pick up the phone and phone the Varsity and ask to speak to the relevant Prof - normally they are very happy to help if you approach them civilly and explain your problem.